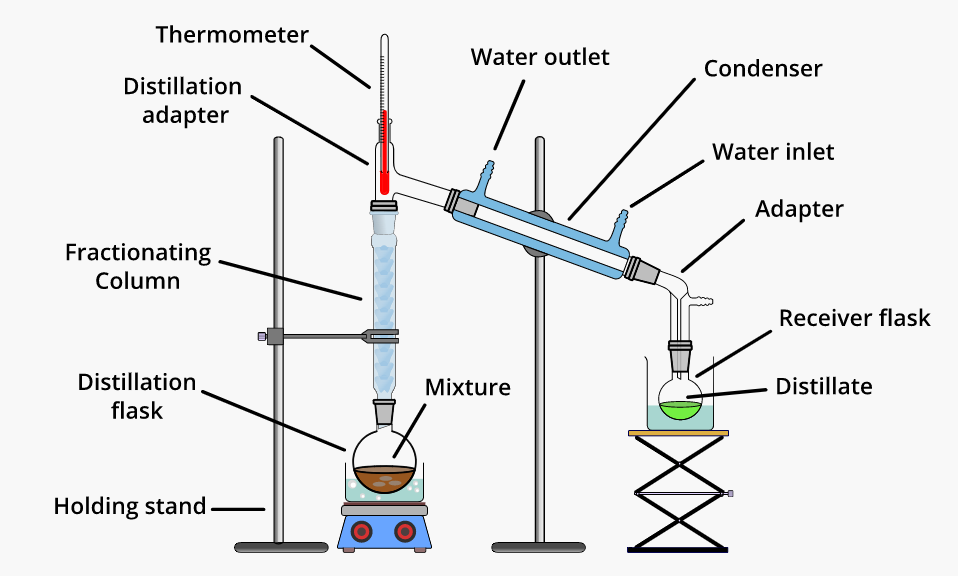
Fractional distillation is the solvent recycling process where solvent purification and oil refining is done. This process is used to separate the complex mixtures for reusing. The method works when the mixed components have different boiling points, allowing one to vaporize before others.
It doesn’t need manual labor, so it is more efficient than other solvent recovery methods. Fractional distillation also reduces hazardous waste, resulting in less need for waste removal.
Sections
ToggleUnderstanding Solvent Recovery and Fractional Distillation
Fractional distillation is crucial when trying to separate and extract the chemical compounds from the solution. The method can be used to recover solvents, thus allowing for a cost effective way of reusing the solvents rather than buying new virgin products.
Why is Fractional Distillation More Efficient?
Common waste solvents such as MEK, acetone, line wash, lacquer thinners, gun wash, IPA and more are burned as fuel. As environmental waste has become a big issue, the benefits of recycling and recovery outweigh the burning part. Using fractional distillation and specifically engineered equipment to speed up the whole process, companies can take the waste they produce and recycle, reusing them as new products. The businesses can thus be able to save cost.
- Precise separation:Fractional distillation helps isolate certain components in the mixture, which leads to precise separation of the solvents and components.
- Better yield: Fractional distillation leads to purer products but lesser yield due to the ratio of 20 per cent ethanol to water. The fact that the yield is low indicates that the distillate is better and pure.
- Energy efficiency: This method of solvent recovery is more efficient than simple distillation as it produces purer distillate and saves energy to a large extent.
- Reduces contamination: Due to its unique distillation process, the fractional method further reduces the chances of contamination, thus providing purer distillate.
- Scalability: Fractional distillation can be applied to numerous mixtures with a wide range of boiling points.
Fractional Vs. Other Solvent Recovery Methods
1. Simple distillation
- Simple distillation is the basic distillation method, suitable to separate liquids with different boiling points.
- Fractional distillation is the method of separating mixtures of liquids that have closed boiling points.
2. Rotary evaporation
- Rotary evaporation reduces the volume of solvents by distributing them as thin film across a vessel’s interior at high temperature and less pressure.
- Fractional distillation is the process used in separation of miscible liquids, requiring repeated distillations as well as condensations of the mixture.
3. Membrane filtration
- Membrane filtration is used to separate molecules or particles from a gas or liquid using the semi permeable membrane.
- Fractional distillation method is used for separating the components of a liquid mixture in pure form.
Key Industries Benefiting from Fractional Distillation
Fractional distillation is a true game changer for a lot of industries that deal with complex chemical mixtures like cosmetics, pharmaceuticals, oil and gas and food production.
Pharmaceuticals
- Pharma companies purify the medical compounds and ingredients using distillation.
- Automated monitoring helps to track the solvent quality all through the recovery process
- Distillation systems maintain the purity of the product at molecular level
- The validation protocols ensure pharmaceutical grade solvents quality
Cannabis and extraction
- Beverage producers make spirits of certain alcohol content using fractional distillation.
- Fractional distillation is used for separating and purifying cannabinoids in the later stage of creating cannabis oil extracts.
- The process takes place after cannabis materials have undergone the winterization and decaorboxylation steps.
Chemical manufacturing
- Chemical manufacturing units purify the raw materials to produce plastics, industrial and pharmaceutical solvents.
- Filtration removes the contaminants from the solvents.
- Specialized materials help to handle the reactive and corrosive solvent mixtures.
- The integrated control solutions optimize recovery rate in chemical production.
Petrochemical
- The petroleum industry separates crude oil in diesel, gasoline and other products.
- Oil refineries process huge amounts of barrels daily through distillation columns.
- Separation methods maintain solvent quality and the automated controls adjust the parameters for various solvents used in the petrochemical industry.
- Customized recovery methods handle coating solvent mixtures
Conclusion
With solvent recovery, fractional distillation is both environmentally friendly and cost-efficient. With its ability to separate the solvent mixtures used previously, it further contributes to purifying and cleaning them to provide a solution that can be used repeatedly.
Fractional distillation is essential for modern industrial processes across different manufacturing sectors. This is an advanced process that separates various solvents efficiently, each with different concentrations, densities and boiling points. Thus, fractional distillation is important in industries where purity of the chemical compounds is crucial.




